Deep Dive: The 12 Hallmarks of Aging — Complete Guide¶
Reading time: ~20 minutes
Prerequisite: Chapter 1.2
The Big Picture¶
In 2013, a team of leading aging researchers published a landmark paper identifying the core biological processes that drive aging at the cellular level. They called these the "hallmarks of aging." In 2023, the framework was updated to 12 hallmarks based on a decade of research.¹
This deep dive covers all 12 hallmarks in detail. You don't need to memorize all of this. It's here as a reference when you want to go deeper. The main chapter covers the 3 most coachable hallmarks (mitochondrial dysfunction, inflammaging, and autophagy). This guide gives you the complete picture.
How Hallmarks Interconnect¶
Before we dive into each hallmark, understand that they're not isolated. They form a network where one process influences others.
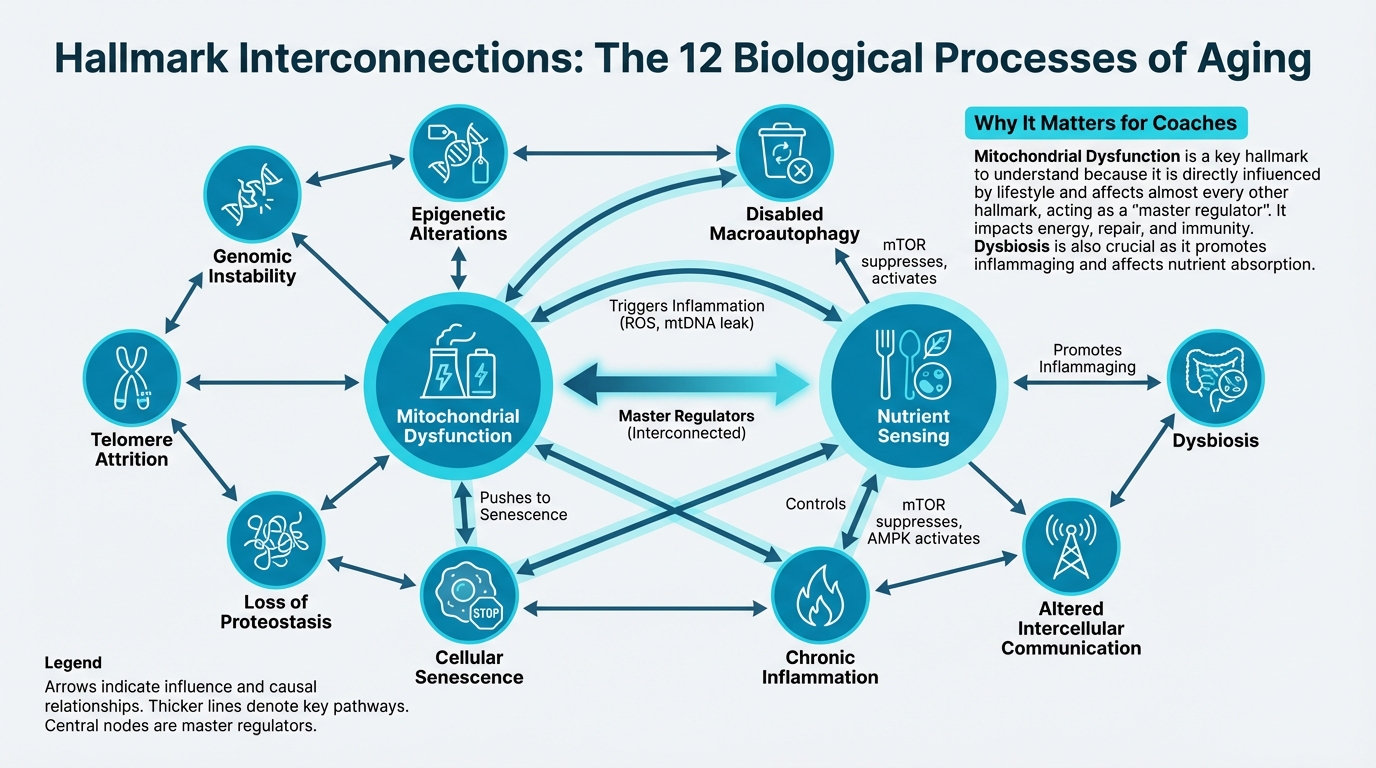
Figure: How hallmarks influence each other
Key interconnections:
Mitochondrial dysfunction → Inflammation → Senescence: When mitochondria don't work well, they leak harmful molecules that trigger inflammation. This inflammation can push cells into senescence (zombie cells). Senescent cells then produce more inflammatory signals, creating a vicious cycle.
Nutrient sensing → Autophagy → Proteostasis: When nutrient-sensing pathways (like mTOR) are overactive, they suppress autophagy (cellular cleanup). This leads to accumulation of damaged proteins, disrupting protein quality control.
Epigenetic alterations → Genomic instability → Senescence: Changes in how genes are turned on/off can lead to DNA damage, which can trigger cells to become senescent. Senescent cells then produce signals that further alter gene expression.
The takeaway: You can't think about aging as 12 separate problems. That's why interventions targeting multiple hallmarks (like exercise) tend to be more effective than single-target interventions.
The 12 Hallmarks¶
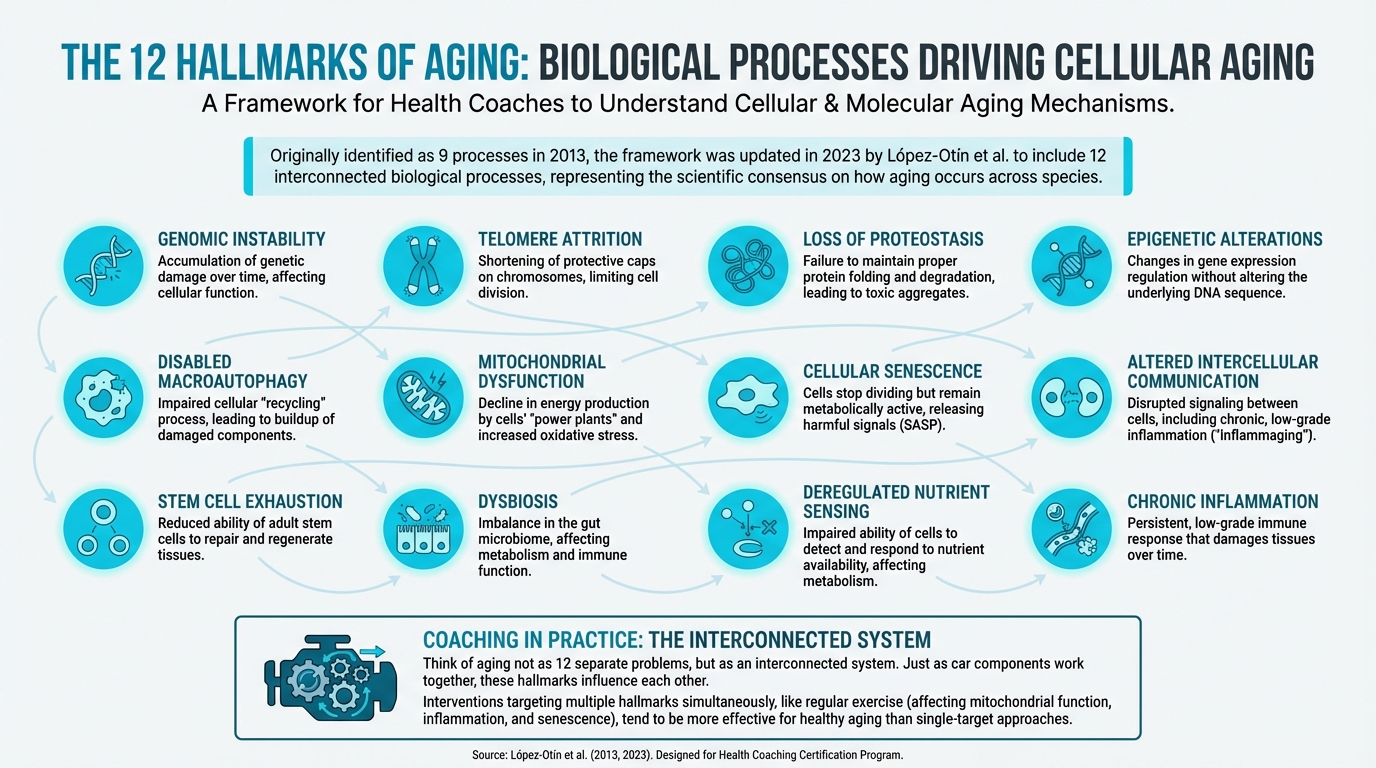
Figure: Visual showing all 12 hallmarks with icons
1. Genomic Instability¶
What it is: DNA damage accumulates over time. Your DNA is constantly being damaged by UV light, radiation, chemicals, and normal cellular processes. Usually, your cells repair this damage. But as you age, repair systems become less efficient, and damage accumulates.
The analogy: DNA is like a book. Over time, pages get torn, words get smudged, and errors creep in. Your cells have "editors" (repair enzymes) that fix errors. But as you age, the editors get slower and make more mistakes.
Why it matters: Accumulated DNA damage can lead to cancer, cellular dysfunction, and cell death.
What coaches can influence: Exercise reduces DNA damage markers. A 2018 review found that resistance training reduced markers of genomic instability by about 15-20%.² Good nutrition (antioxidants, adequate protein for repair enzymes) and avoiding toxins (smoking, excessive alcohol) also help.
2. Telomere Attrition¶
What it is: Telomeres are protective caps on the ends of your chromosomes, like the plastic tips on shoelaces. Every time a cell divides, telomeres get shorter. Eventually, they get too short, and the cell stops dividing or becomes senescent.
The analogy: Telomeres are like shoelace tips. Each time you tie your shoes, the tips wear down. Eventually, they wear away completely, and the lace starts to fray.
Why it matters: Short telomeres are associated with increased risk of age-related diseases and earlier mortality.
What coaches can influence: Exercise maintains telomere length. A 2025 meta-analysis found that exercise significantly maintained telomere length and increased telomerase activity.³ The effect was strongest with interventions lasting 16 weeks or more. Stress management, good sleep, and social connection also preserve telomeres.
3. Epigenetic Alterations¶
What it is: Modifications to your DNA that turn genes on or off without changing the DNA sequence itself. Think of it like volume controls on your genes. As you age, these "volume controls" get messed up.
The analogy: Your DNA is like a piano. The keys (genes) are the same, but the sheet music (epigenetic marks) tells you which keys to play. As you age, the sheet music gets smudged.
Why it matters: Epigenetic changes affect how your cells function. Researchers can estimate your "biological age" by looking at epigenetic patterns. These "epigenetic clocks" predict health outcomes better than chronological age alone.
What coaches can influence: Lifestyle interventions can slow epigenetic aging. A 2023 study found that two years of moderate caloric restriction slowed epigenetic aging by about 2-3%.⁴ Exercise, good sleep, stress management, and avoiding smoking also help.
For more detail, see Deep Dive: Hallmark Deep Dive: Epigenetic Alterations
4. Loss of Proteostasis¶
What it is: Proteostasis is your cells' system for maintaining protein quality. Proteins need to be folded correctly to work. When they misfold, your cells have cleanup systems that break them down. With age, these systems become less efficient.
The analogy: Proteins are like origami. They need to be folded into the right shape. Your cells have "quality control inspectors" that check the folding and recycle misfolded proteins. As you age, the inspectors get slower.
Why it matters: Accumulated misfolded proteins are toxic and associated with neurodegenerative diseases (Alzheimer's, Parkinson's).
What coaches can influence: Exercise, caloric restriction, and good sleep all support proteostasis. Autophagy (cellular cleanup) is enhanced by exercise and fasting.
5. Disabled Macroautophagy¶
What it is: Autophagy ("self-eating") is your cells' recycling system. It breaks down damaged cellular components and recycles them. With age, autophagy becomes less efficient.
The analogy: Autophagy is like a cellular recycling center. Damaged parts get broken down and turned into new materials. As you age, the recycling center gets slower.
Why it matters: When autophagy slows down, damaged proteins, organelles, and other cellular junk accumulate.
What coaches can influence: Exercise, caloric restriction, and time-restricted eating all enhance autophagy. Fasting is particularly powerful. When you don't eat for 12-16 hours, cells ramp up autophagy. A 2025 study found that 6 months of time-restricted eating increased markers of autophagy.⁵
6. Deregulated Nutrient Sensing¶
What it is: Your cells have sensors that detect nutrients and energy availability. Two key pathways are mTOR (signals "nutrients available, grow") and AMPK (signals "low energy, focus on repair"). With age, these pathways become imbalanced.
The analogy: mTOR and AMPK are like a seesaw. When nutrients are abundant, mTOR is up (growth). When energy is low, AMPK is up (maintenance). With age, the seesaw gets stuck, mTOR stays up even when it shouldn't.
Why it matters: Overactive mTOR accelerates aging. Restoring balance extends lifespan in many species.
What coaches can influence: Caloric restriction, time-restricted eating, and protein moderation all lower mTOR and activate AMPK. Exercise also activates AMPK.
Deeper explanation:
mTOR (mechanistic target of rapamycin) signals cells to:
- Grow and divide
- Make new proteins
- Store energy
- Suppress autophagy (cleanup)
AMPK (AMP-activated protein kinase) signals cells to:
- Activate autophagy
- Improve mitochondrial function
- Reduce inflammation
- Enhance DNA repair
7. Mitochondrial Dysfunction¶
What it is: Mitochondria are your cells' power plants. They produce energy (ATP) from food and oxygen. With age, mitochondria become less efficient, produce less energy, and leak harmful molecules.
The analogy: Mitochondria are like power plants. They take fuel and turn it into electricity. As you age, the power plants get less efficient, produce less electricity, and leak pollution.
Why it matters: Mitochondrial dysfunction affects every cell. It triggers inflammation, causes senescence, and disrupts nutrient sensing.
What coaches can influence: Exercise is the #1 intervention. A 2024 meta-analysis found that endurance training increases mitochondrial content by about 23%, and HIIT by about 27%.⁶ Sleep and circadian alignment also matter.
8. Cellular Senescence¶
What it is: Senescent cells are "zombie cells". They've stopped dividing but haven't died. They accumulate with age and produce harmful signals (SASP) that cause inflammation and damage surrounding cells.
The analogy: Senescent cells are like broken-down cars abandoned on the road. They're not moving, but they're leaking oil and blocking traffic.
Why it matters: Senescent cells are a major driver of aging. Clearing them extends lifespan in mice.
What coaches can influence: Exercise reduces senescent cells. A 2021 study found that 12 weeks of exercise lowered senescence markers.⁷ A 2024 study found that two years of caloric restriction significantly reduced multiple senescence biomarkers.⁸
For more detail, see Deep Dive: Hallmark Deep Dive: Cellular Senescence
9. Stem Cell Exhaustion¶
What it is: Stem cells are your body's repair crew. They can become other cell types. With age, stem cells become depleted and less functional.
The analogy: Stem cells are like a construction crew that can build any type of building. As you age, the crew gets smaller and less skilled.
Why it matters: When stem cells are exhausted, your body can't repair and regenerate tissue as well. This contributes to muscle loss, skin aging, and impaired healing.
What coaches can influence: Exercise preserves stem cell function. Resistance training helps maintain muscle stem cells. Caloric restriction can also preserve stem cell populations.
10. Altered Intercellular Communication¶
What it is: Cells communicate through chemical signals (hormones, cytokines). With age, this communication breaks down: too much of some signals (inflammatory), too little of others (growth factors).
The analogy: Cells are like people in an office who need to communicate. As the office ages, communication breaks down. Some people send too many emails, others stop responding.
Why it matters: When cell-to-cell communication breaks down, tissues can't coordinate properly.
What coaches can influence: Exercise, good nutrition, and stress management all help restore healthier cell signaling. Social connection also supports better intercellular communication.
11. Chronic Inflammation (Inflammaging)¶
What it is: With age, your body develops persistent, low-grade inflammation. It's not the acute inflammation from an injury (that's helpful). It's chronic inflammation that never fully resolves.
The analogy: Acute inflammation is like a fire alarm. Inflammaging is like a smoke detector with a low battery that beeps constantly, not helpful, just draining.
Why it matters: Chronic inflammation accelerates all the other hallmarks of aging.
What coaches can influence: Exercise, Mediterranean-style nutrition, good sleep, stress management, and social connection all reduce inflammaging.
12. Dysbiosis¶
What it is: An imbalance in your microbial communities, especially your gut microbiome. With age, beneficial bacteria decline and harmful bacteria increase.
The analogy: Your gut microbiome is like a garden. When healthy, you have diverse beneficial plants. With age, weeds take over.
Why it matters: Your gut microbiome affects inflammation, immune function, nutrient absorption, and brain health.
What coaches can influence: Diet is the biggest lever. Fiber-rich, diverse, plant-based foods support a healthy microbiome. Exercise and good sleep also help.
Interventions × Hallmarks Matrix¶
Which interventions affect which hallmarks? Here's a comprehensive reference:
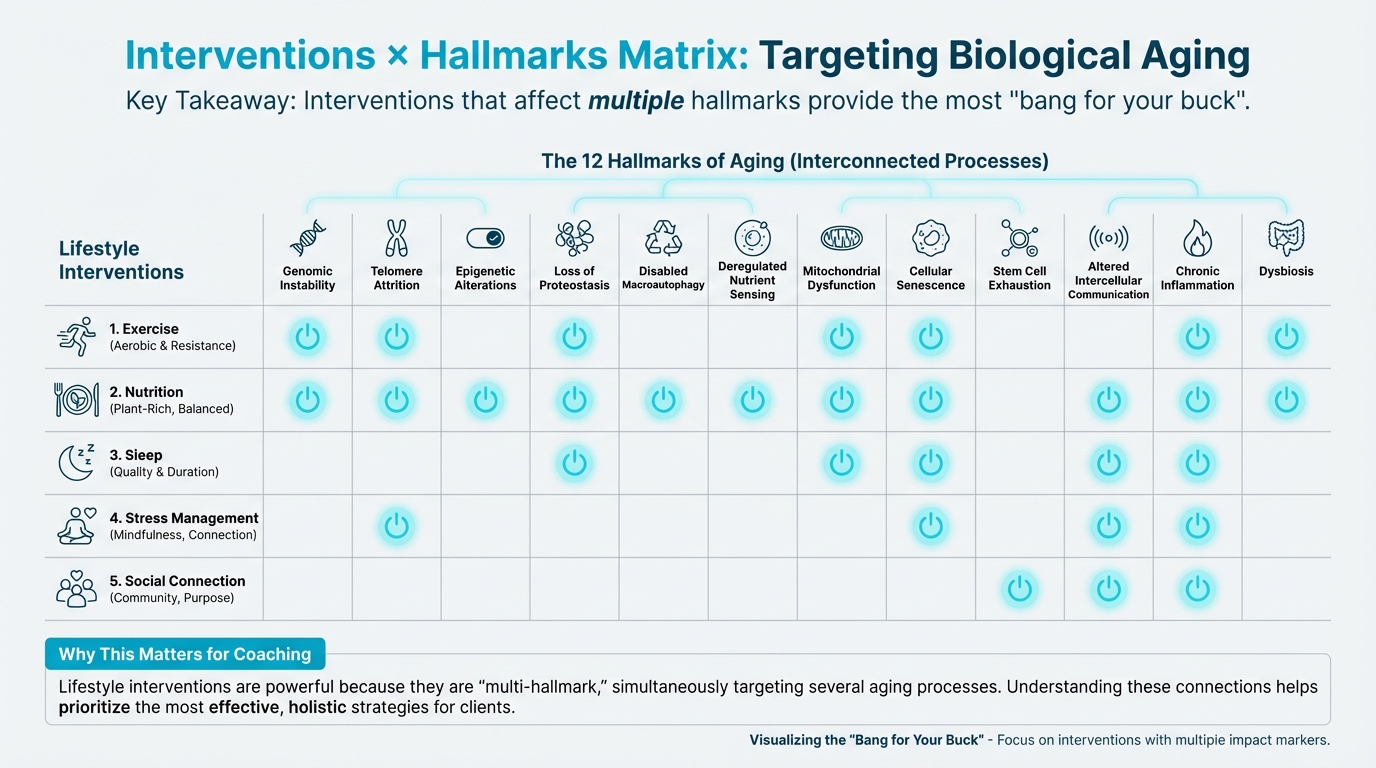
Figure: Which interventions affect which hallmarks
| Intervention | Genomic Instability | Telomere Attrition | Epigenetic | Proteostasis | Autophagy | Nutrient Sensing | Mitochondria | Senescence | Stem Cells | Communication | Inflammaging | Dysbiosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise | Strong | Strong | Moderate | Moderate | Strong | Strong | Strong | Strong | Moderate | Moderate | Strong | Weak |
| Mediterranean Diet | Moderate | Moderate | Moderate | Moderate | Moderate | Strong | Moderate | Moderate | Weak | Moderate | Strong | Strong |
| Sleep | Moderate | Moderate | Moderate | Strong | Strong | Moderate | Moderate | Moderate | Moderate | Moderate | Strong | Moderate |
| Stress Management | Moderate | Strong | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate | Weak | Moderate | Strong | Weak |
| Social Connection | Weak | Moderate | Weak | Weak | Weak | Weak | Weak | Weak | Weak | Moderate | Strong | Weak |
| Caloric Restriction | Moderate | Moderate | Strong | Moderate | Strong | Strong | Moderate | Strong | Moderate | Moderate | Moderate | Moderate |
| Time-Restricted Eating | Weak | Weak | Weak | Moderate | Strong | Strong | Moderate | Weak | Weak | Weak | Moderate | Moderate |
Legend: Strong = robust evidence; Moderate = some evidence; Weak = limited evidence
What This Means for Coaches¶
-
Exercise is the ultimate multi-hallmark intervention. It affects 10 out of 12 hallmarks strongly or moderately. This is why exercise is often called "the longevity drug."
-
Mediterranean diet targets multiple hallmarks, especially nutrient sensing, inflammaging, and dysbiosis.
-
Sleep affects many hallmarks, especially proteostasis, autophagy, and inflammaging. Poor sleep accelerates aging across multiple systems.
-
Focus on interventions that target multiple hallmarks: the fundamentals (exercise, nutrition, sleep, stress management) work because they hit multiple targets simultaneously.
-
You don't need to explain all 12 to clients: focus on the 3 Big Levers (mitochondria, inflammation, autophagy) and reference this guide when you need more depth.
Key Takeaway¶
The 12 hallmarks of aging are interconnected biological processes that drive aging at the cellular level. Lifestyle interventions work because they target multiple hallmarks simultaneously, especially exercise, which affects 10 of the 12.
References¶
-
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023;186(2):243-278. doi:10.1016/j.cell.2022.11.001
-
Rebelo-Marques A, De Sousa Lages A, Andrade R, et al. Aging Hallmarks: The Benefits of Physical Exercise. Frontiers in Endocrinology. 2018;9. doi:10.3389/fendo.2018.00258
-
Sun L, Zhang T, Luo L, et al. Exercise delays aging: evidence from telomeres and telomerase. Frontiers in Physiology. 2025;16. doi:10.3389/fphys.2025.1627292
-
Waziry R, Corcoran D, Huffman K, et al. Effect of Long-Term Caloric Restriction on DNA Methylation Measures of Biological Aging. 2021. doi:10.1101/2021.09.21.21263912
-
Bensalem J, Teong XT, Hattersley KJ, et al. Intermittent time‐restricted eating may increase autophagic flux in humans. The Journal of Physiology. 2025;603(10):3019-3032. doi:10.1113/jp287938
-
Mølmen KS, Almquist NW, Skattebo Ø. Effects of Exercise Training on Mitochondrial and Capillary Growth in Human Skeletal Muscle. Sports Medicine. 2024;55(1):115-144. doi:10.1007/s40279-024-02120-2
-
Englund DA, Sakamoto AE, Fritsche CM, et al. Exercise reduces circulating biomarkers of cellular senescence in humans. Aging Cell. 2021;20(7). doi:10.1111/acel.13415
-
Aversa Z, White TA, Heeren AA, et al. Calorie restriction reduces biomarkers of cellular senescence in humans. Aging Cell. 2023;23(2). doi:10.1111/acel.14038